
中国农学通报 ›› 2021, Vol. 37 ›› Issue (17): 120-128.doi: 10.11924/j.issn.1000-6850.casb2020-0480
姜硕1,2( ), 万璐1,2, 许哲祥1,2, 闫佳佳1,2, 郑春英1,2(
), 万璐1,2, 许哲祥1,2, 闫佳佳1,2, 郑春英1,2( )
)
收稿日期:2020-09-20
修回日期:2020-12-08
出版日期:2021-06-15
发布日期:2021-06-29
通讯作者:
郑春英
作者简介:姜硕,女,1997年出生,黑龙江大庆人,硕士研究生,研究方向:食品和药物生物活性挖掘及研发。通信地址:150080 黑龙江省哈尔滨市南岗区学府路74号 黑龙江大学生命科学学院,Tel:0451-86608586,E-mail:基金资助:
Jiang Shuo1,2( ), Wan Lu1,2, Xu Zhexiang1,2, Yan Jiajia1,2, Zheng Chunying1,2(
), Wan Lu1,2, Xu Zhexiang1,2, Yan Jiajia1,2, Zheng Chunying1,2( )
)
Received:2020-09-20
Revised:2020-12-08
Online:2021-06-15
Published:2021-06-29
Contact:
Zheng Chunying
摘要:
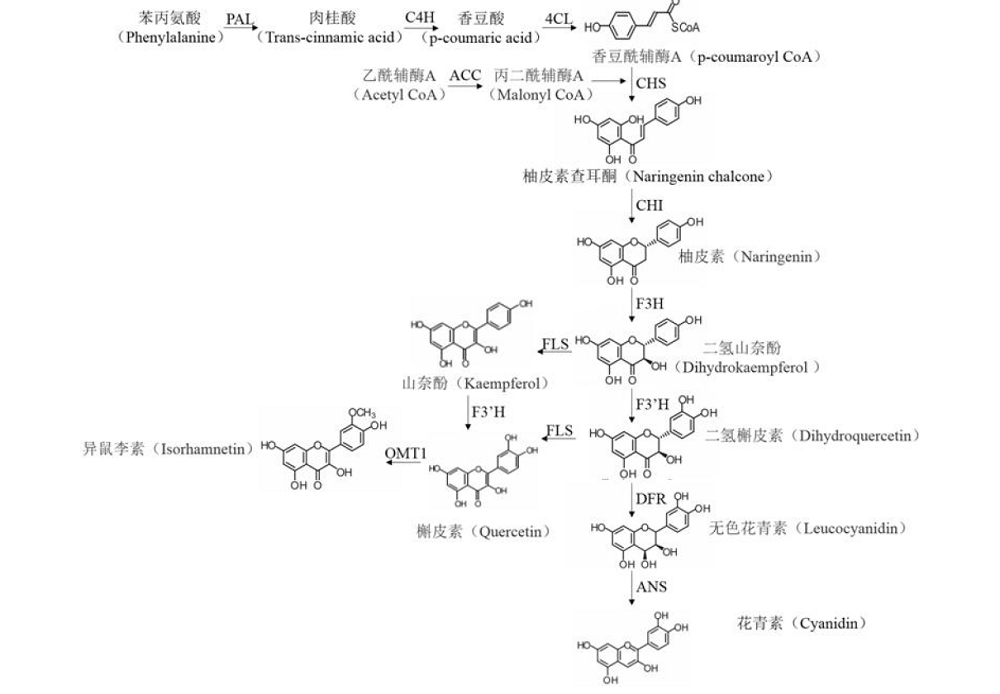
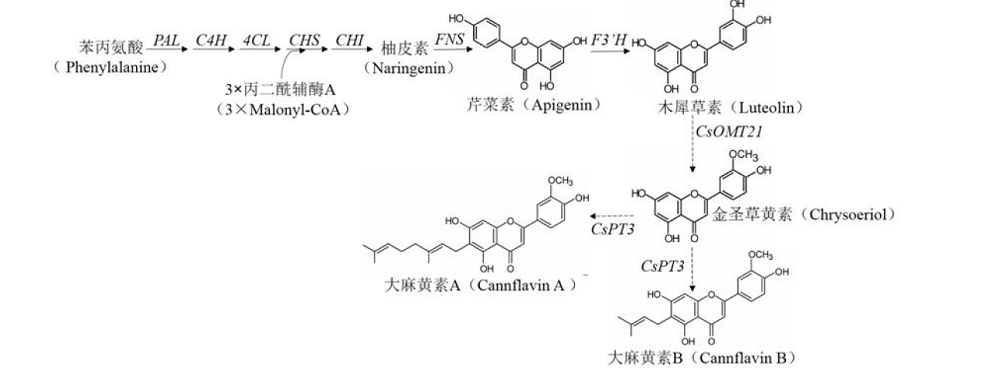
为了高效利用汉麻黄酮类成分及系统开展汉麻黄酮类化合物的研究,本文综述了汉麻黄酮类化合物的结构、合成途径、生物活性及分析方法。归纳总结出汉麻中共含有26种黄酮类化合物,其主要结构类型以黄酮和黄酮醇为主;此外,在黄酮类化合物经典合成途径的基础上,明确了2种特征性汉麻黄酮成分-大麻黄素A和大麻黄素B的生物合成途径;同时,也总结了汉麻黄酮类化合物具有抗肿瘤、神经保护、抗抑郁等生物活性,并针对其结构特点和活性作用,建立了汉麻黄酮类化合物高效、快速的分析方法为LC-MS等功效。上述归纳可为开展汉麻黄酮类化合物的研究及应用提供参考。
中图分类号:
姜硕, 万璐, 许哲祥, 闫佳佳, 郑春英. 汉麻黄酮类成分研究进展[J]. 中国农学通报, 2021, 37(17): 120-128.
Jiang Shuo, Wan Lu, Xu Zhexiang, Yan Jiajia, Zheng Chunying. Research Progress on Flavonoids of Cannabis sativa L[J]. Chinese Agricultural Science Bulletin, 2021, 37(17): 120-128.
| 序号 | 名称 | 分子式 | 结构式 | 文献来源 |
|---|---|---|---|---|
| 1 | 芹菜素 (Apigenin) | C15H10O5 |  | Delgado, et al.[ |
| 2 | 槲皮素 (Quercetin) | C15H10O7 |  | Delgado, et al.[ |
| 3 | 槲皮素-3-半乳糖苷 (Quercetin-3-galactoside) | C21H20O12 |  | Delgado, et al.[ |
| 4 | 山奈酚-7-O-葡萄糖苷 (Kaempferol-7-O-glucoside) | C21H20O10 |  | Delgado, et al.[ |
| 5 | 原花青素B2 (Procyanidin B2) | C30H26O12 |  | Delgado, et al.[ |
| 6 | 牡荆素-2"-O-α-L-鼠李糖苷 (vitexin-2"-O-α-L-rhamnoside) | C27H30O14 |  | Delgado, et al.[ |
| 7 | 异荭草素 (Luteolin-6-C-glucoside) | C21H20O11 |  | Delgado, et al.[ |
| 序号 | 名称 | 分子式 | 结构式 | 文献来源 |
| 8 | 香叶木素 (Diosmetin) | C16H12O6 |  | Delgado, et al.[ |
| 9 | 牡荆素 (Vitexin) | C21H20O10 |  | Delgado, et al.[ |
| 10 | 大麻黄素C (Cannflavin C) | C26H28O6 |  | Radwan, et al.[ |
| 11 | 异戊烯基芹菜素 (6-Prenylapigenin) | C20H18O5 |  | Radwan, et al.[ |
| 12 | 金圣草黄素 (Chrysoeriol) | C16H12O6 |  | Kevin, et al.[ |
| 13 | 大麻黄素A (Cannflavin A) | C26H28O6 |  | Kevin, et al.[ |
| 14 | 大麻黄素B (Cannflavin B) | C21H20O6 |  | Kevin, et al.[ |
| 15 | 木犀草素 (Luteolin) | C15H10O6 |  | Kevin, et al.[ |
| 16 | 芹菜素7-O-葡萄糖苷 (Apigenin 7-O-glucoside) | C21H20O10 |  | 成亮,等.[ |
| 17 | 木犀草素-7-O-β-D-吡喃葡萄糖苷 (Luteolin-7-O-β-D-glucopyranoside) | C21H20O11 |  | 成亮,等.[ |
| 18 | 芦丁 (Rutin) | C27H30O16 |  | 成亮,等.[ |
| 序号 | 名称 | 分子式 | 结构式 | 文献来源 |
| 19 | 槲皮素-3-O-α-L-鼠李糖苷 (Quercetin-3-O-α-L-rhamnoside) | C27H30O16 |  | 成亮,等.[ |
| 20 | 芹菜素-6,8-二-C-β-D-吡喃葡萄糖苷 (Apigenin-6,8-di-C-β-D-glucopyranoside) | C27H30O16 |  | 成亮,等.[ |
| 21 | 山奈酚-3-O-α-L-鼠李糖苷 (Kaempferol-3-O-α-L-rhamnoside) | C21H20O10 |  | 成亮, 等.[ |
| 22 | 荭草素 (Orientin) | C21H20O11 |  | 成亮,等.[ |
| 23 | 异牡荆素 (Isovitexi) | C21H20O10 |  | Amany, et al.[ |
| 序号 | 名称 | 分子式 | 结构式 | 文献来源 |
|---|---|---|---|---|
| 1 | 芹菜素 (Apigenin) | C15H10O5 |  | Delgado, et al.[ |
| 2 | 槲皮素 (Quercetin) | C15H10O7 |  | Delgado, et al.[ |
| 3 | 槲皮素-3-半乳糖苷 (Quercetin-3-galactoside) | C21H20O12 |  | Delgado, et al.[ |
| 4 | 山奈酚-7-O-葡萄糖苷 (Kaempferol-7-O-glucoside) | C21H20O10 |  | Delgado, et al.[ |
| 5 | 原花青素B2 (Procyanidin B2) | C30H26O12 |  | Delgado, et al.[ |
| 6 | 牡荆素-2"-O-α-L-鼠李糖苷 (vitexin-2"-O-α-L-rhamnoside) | C27H30O14 |  | Delgado, et al.[ |
| 7 | 异荭草素 (Luteolin-6-C-glucoside) | C21H20O11 |  | Delgado, et al.[ |
| 序号 | 名称 | 分子式 | 结构式 | 文献来源 |
| 8 | 香叶木素 (Diosmetin) | C16H12O6 |  | Delgado, et al.[ |
| 9 | 牡荆素 (Vitexin) | C21H20O10 |  | Delgado, et al.[ |
| 10 | 大麻黄素C (Cannflavin C) | C26H28O6 |  | Radwan, et al.[ |
| 11 | 异戊烯基芹菜素 (6-Prenylapigenin) | C20H18O5 |  | Radwan, et al.[ |
| 12 | 金圣草黄素 (Chrysoeriol) | C16H12O6 |  | Kevin, et al.[ |
| 13 | 大麻黄素A (Cannflavin A) | C26H28O6 |  | Kevin, et al.[ |
| 14 | 大麻黄素B (Cannflavin B) | C21H20O6 |  | Kevin, et al.[ |
| 15 | 木犀草素 (Luteolin) | C15H10O6 |  | Kevin, et al.[ |
| 16 | 芹菜素7-O-葡萄糖苷 (Apigenin 7-O-glucoside) | C21H20O10 |  | 成亮,等.[ |
| 17 | 木犀草素-7-O-β-D-吡喃葡萄糖苷 (Luteolin-7-O-β-D-glucopyranoside) | C21H20O11 |  | 成亮,等.[ |
| 18 | 芦丁 (Rutin) | C27H30O16 |  | 成亮,等.[ |
| 序号 | 名称 | 分子式 | 结构式 | 文献来源 |
| 19 | 槲皮素-3-O-α-L-鼠李糖苷 (Quercetin-3-O-α-L-rhamnoside) | C27H30O16 |  | 成亮,等.[ |
| 20 | 芹菜素-6,8-二-C-β-D-吡喃葡萄糖苷 (Apigenin-6,8-di-C-β-D-glucopyranoside) | C27H30O16 |  | 成亮,等.[ |
| 21 | 山奈酚-3-O-α-L-鼠李糖苷 (Kaempferol-3-O-α-L-rhamnoside) | C21H20O10 |  | 成亮, 等.[ |
| 22 | 荭草素 (Orientin) | C21H20O11 |  | 成亮,等.[ |
| 23 | 异牡荆素 (Isovitexi) | C21H20O10 |  | Amany, et al.[ |

| 序号 | 方法 | 条件 | 文献来源 |
|---|---|---|---|
| 1 | HPLC-UV (梯度洗脱) | 流动相:溶剂A:水(0.1%TFA);溶剂B:水-乙腈(65:35,TFA 0.1%) 溶剂C:乙腈;检测波长:254 nm | Wieland, et al[ |
| 2 | HPLC-MS (梯度洗脱) | 流动相:溶剂A:5%乙腈-0.1% FA;溶剂B:乙腈-5%水-0.1% FA; 离子源:电喷雾离子源(ESI);检测方式:正负离子模式检测 | Delgado, et al[ |
| 3 | HPLC-MS (梯度洗脱) | 流动相:溶剂A:1%甲酸-水;溶剂B:5%乙腈-甲醇; 离子源:大气压电喷雾离子源(API-ESI);检测方式:正离子模式检测 | Vanhoenacke, et al[ |
| 序号 | 方法 | 条件 | 文献来源 |
|---|---|---|---|
| 1 | HPLC-UV (梯度洗脱) | 流动相:溶剂A:水(0.1%TFA);溶剂B:水-乙腈(65:35,TFA 0.1%) 溶剂C:乙腈;检测波长:254 nm | Wieland, et al[ |
| 2 | HPLC-MS (梯度洗脱) | 流动相:溶剂A:5%乙腈-0.1% FA;溶剂B:乙腈-5%水-0.1% FA; 离子源:电喷雾离子源(ESI);检测方式:正负离子模式检测 | Delgado, et al[ |
| 3 | HPLC-MS (梯度洗脱) | 流动相:溶剂A:1%甲酸-水;溶剂B:5%乙腈-甲醇; 离子源:大气压电喷雾离子源(API-ESI);检测方式:正离子模式检测 | Vanhoenacke, et al[ |
| [1] | Romero P, Peris A, Vergara K, et al. Comprehending and improving cannabis specialized metabolism in the systems biology era[J]. Plant Science, 2020,298:e110571. |
| [2] | 国家药典委员会. 中华人民共和国药典 2020年版(一部)[S]. 北京: 中国医药科技出版社, 2020: 81. |
| [3] |
Tahseen K, Singh M. The application of hemp (Cannabis sativa L.) for a green economy: a review[J]. Turkish Journal of Botany, 2019,43:710-723.
doi: 10.3906/bot-1907-15 |
| [4] | 郭蓉, 陈璇, 郭鸿彦. 四氢大麻酚和大麻二酚的药理研究进展[J]. 天然产物研究与开发, 2017,29(8):1449-1453. |
| [5] |
Sexton M, Shelton K, Haley P, et al. Evaluation of cannabinoid and terpenoid content: cannabis flower compared to supercritical CO2 concentrate[J]. Planta Med, 2018,84:234-241.
doi: 10.1055/s-0043-119361 URL |
| [6] |
Finnan J, Styles D. Hemp: a more sustainable annual energy crop for climate and energy policy[J]. Energy Policy, 2013,58:152-162.
doi: 10.1016/j.enpol.2013.02.046 URL |
| [7] |
Amaducci S, Zatta A, Raffanini M, et al. Characterisation of hemp (Cannabis sativa L.) roots under different growing conditions[J]. Plant Soil, 2008,313:227-235.
doi: 10.1007/s11104-008-9695-0 URL |
| [8] |
Vukčević M M, Kalijadis A M, Vasiljević T M, et al. Production of activated carbon derived from waste hemp (Cannabis sativa) fibers and its performance in pesticide adsorption[J]. Microporous Mesoporous Mater, 2015,214:156-165.
doi: 10.1016/j.micromeso.2015.05.012 URL |
| [9] |
Virginia B, Federica P, Marleen S, et al. Development of a new extraction technique and HPLC method for the analysis of non-psychoactive cannabinoids in fibre-type Cannabis sativa L[J]. Journal of Pharmaceutical and Biomedical Analysis, 2017,143:228-236.
doi: 10.1016/j.jpba.2017.05.049 URL |
| [10] |
Sara A B, Marika P, Simone T, et al. Cannabis sativa: A comprehensive ethnopharmacological review of a medicinal plant with a long history[J]. Journal of Ethnopharmacology, 2018,227:300-315.
doi: 10.1016/j.jep.2018.09.004 URL |
| [11] |
Pellati F, Brighenti V, Sperlea J, et al. New Methods for the Comprehensive Analysis of Bioactive Compounds in Cannabis sativa L (hemp)[J]. Molecules, 2018,23(10):2639-2660.
doi: 10.3390/molecules23102639 URL |
| [12] | Julia D A, Marcos V L, Raphael C, et al. Extraction and isolation of cannabinoids from marijuana seizures and characterization by 1H NMR allied to chemometric tools[J]. Science & Justice, 2018,58(5):355-365. |
| [13] |
Ma Q, Jiang J G, Yuan X H, et al. Comparative antitumor and anti-inflammatory effects of flavonoids, saponins, polysaccharides, essential oil, coumarin and alkaloids from Cirsium japonicum DC[J]. Food and Chemical Toxicology, 2019,125:422-429.
doi: 10.1016/j.fct.2019.01.020 URL |
| [14] |
Xu F, Wang C X, Wang H F, et al. Antimicrobial action of flavonoids from Sedum aizoon L. against lactic acid bacteria in vitro and in refrigerated fresh pork meat[J]. Journal of Functional Foods, 2018,40:744-750.
doi: 10.1016/j.jff.2017.09.030 URL |
| [15] |
Chen G L, Fan M X, Wu J L, et al. Antioxidant and anti-inflammatory properties of flavonoids from lotus plumule[J]. Food Chemistry, 2019,277:706-712.
doi: 10.1016/j.foodchem.2018.11.040 URL |
| [16] | 胡倩, 刘大会, 曹艳. 艾叶黄酮类化合物的研究进展[J]. 食品安全质量检测学报, 2019,10(12):3648-3653. |
| [17] | Yang X M, Jiang Y M, Yang J L, et al. Prenylated flavonoids, promising nutraceuticals with impressive biological activities[J]. Trends in Food Science & Technology, 2015,44:93-104. |
| [18] | Wang T Y, Li Q, Bi K S. Bioactive flavonoids in medicinal plants: Structure, activity and biological fate[J]. Asian Journal of Pharmaceutical Sciences, 2018,1(13):12-23. |
| [19] |
Saidi, Chawech R, Baccouch N, et al. Study toward antioxidant activity of Clematis flammula extracts: Purification and identification of two flavonoids-glucoside and trisaccharide[J]. South African Journal of Botany, 2019,123:208-213.
doi: 10.1016/j.sajb.2019.03.010 |
| [20] | 孙力, 杨雪冰, 柏广宇, 等. 汉麻活性成分研究现状[J]. 黑龙江科学, 2019,10(6):38-39,42. |
| [21] | Delgado M M, Priego C F, Ferreiro V C, et al. Untargeted characterization of extracts from Cannabis sativa L.cultivars by gas and liquid chromatography coupled to mass spectrometry in high resolution mode[J]. Talanta, 2020,208:e120384. |
| [22] |
Veitch N C, Grayer R J. Flavonoids and their glycosides, including anthocyanins[J]. Natural Product Reports, 2011,28:1626-1695.
doi: 10.1039/c1np00044f pmid: 21845280 |
| [23] |
Ross S A, ElSohly M A, Sultana, et al. Flavonoid glycosides and cannabinoids from the pollen of Cannabis sativa L[J]. Phytochem Anal, 2005,16:45-48.
doi: 10.1002/(ISSN)1099-1565 URL |
| [24] |
Radwan M M, Elsohly M A, Slade D, et al. Non-cannabinoid constituents from a high potency Cannabis sativa variety[J]. Phytochemistry, 2008,69(14):2627-2633.
doi: 10.1016/j.phytochem.2008.07.010 URL |
| [25] |
Kevin A R, Casarettoa M, Sameer A. Biosynjournal of Cannflavins A and B from Cannabis sativa L[J]. Phytochemistry, 2019,164:162-171.
doi: S0031-9422(18)30381-9 pmid: 31151063 |
| [26] | 成亮, 孔德云, 胡光. 工业大麻研究Ⅰ.甲醇提取物的石油醚萃取和正丁醇萃取部分的化学成分[L]. 中国医药工业杂志, 2008,39(1):18-21. |
| [27] | Amany K I, Mohamed M R, Safwat A A, et al. Microbial metabolism of cannflavin A and B isolated from Cannabis sativa[J]. Phytochemistry, 2010,8-9(71):1014-1019. |
| [28] | Cheynier V, Comte G, Davies K M, et al. Plant phenolics: recent advances on their biosynjournal, genetics, and ecophysiology[J]. Plant Physiology and Biochemistry: PPB/Societe francaise de physiologie vegetale, 2013,72:1-20. |
| [29] | Yang B, Liu H L, Yang J L, et al. New insights on bioactivities and biosynjournal of flavonoid glycosides[J]. Trends in Food Science&Technology, 2018,79:116-124. |
| [30] | Saito K, Yonekura K, Nakabayashi R, et al. The flavonoid biosynthetic pathway in Arabidopsis: structural and genetic diversity[J]. Plant Physiology and Biochemistry: PPB/Societe francaise de physiologie vegetale, 2013,72:21-34. |
| [31] |
Wang Y, Chen S, Yu O. Metabolic engineering of flavonoids in plants and microorganisms[J]. Appl Microbiol Biotechnol, 2011,91(4):949-956.
doi: 10.1007/s00253-011-3449-2 URL |
| [32] | Barrett M L, Gordon D, Evans F J. Isolation from cannabis sativa L. of cannflavin-a novel inhibitor of prostaglandin production[J]. Biochemical Pharmacology, 1985,11(34):2019-2024. |
| [33] |
Flores I J, Verpoorte R. PKS activities and biosynjournal of cannabinoids and flavonoids in Cannabis sativa L. plants[J]. Plant Cell Physiol, 2008,49:1767-1782.
doi: 10.1093/pcp/pcn150 URL |
| [34] |
Berim A, Gan D R. Methoxylated flavones: occurrence, importance, biosynjournal[J]. Phytochemistry Rev, 2016,15:363-390.
doi: 10.1007/s11101-015-9426-0 URL |
| [35] | Falcone F, Rius M L, Casati S P. Flavonoids: biosynjournal, biological functions, and biotechnological applications[J]. Plant Sci, 2012,3:222. |
| [36] |
Madunić J, Madunić I V, Gajski G, et al. Apigenin: a dietary flavonoid with diverse anticancer properties[J]. Cancer Lett, 2018,413:11-22.
doi: 10.1016/j.canlet.2017.10.041 URL |
| [37] |
Nabavi S F, Braidy N, Gortzi O, et al. Luteolin as an anti-inflammatory and neuroprotective agent: a brief review[J]. Brain Res Bull, 2015,119:1-11.
doi: 10.1016/j.brainresbull.2015.09.002 URL |
| [38] | 张旭, 高宝昌, 田媛, 等. 汉麻有效成分提取、表征及功能性研究[J]. 黑龙江科学, 2018,9(1):68-69. |
| [39] | 郭萌, 张晴, 闫丽萍, 等. 黄酮类化合物为主要活性成分的单味药和复方中药及其药理作用[J]. 沈阳医学院学报, 2018,20(6):558-561,564. |
| [40] | Sulaiman C T, Arun A, Anandan E M, et al. Isolation and identification of phytoestrogens and flavonoids in an Ayurvedic proprietary medicine using chromatographic and Mass Spectroscopic analysis[J]. Asian Pacific Journal of Reproduction, 2015,2(4):153-156. |
| [41] | Kiss B, Popa D S, Popa D H A, et al. Ultra-performance liquid chromatography method for the quantification of some phytoestrogens in plant material[J]. Rev Roum Chim, 2010,55:459-465. |
| [42] |
Guo T T, Zhang J C, Zhang H. Bioactive spirans and other constituents from the leaves of Cannabis sativa f. sativa[J]. Journal of Asian Natural Products Research, 2016,19(8):793-802.
doi: 10.1080/10286020.2016.1248947 URL |
| [43] |
Liat S, Danielle H S, Noa H, et al. Effects of cannabidiol in males and females in two different rat models of depression[J]. Physiology & Behavior, 2019,201:59-63.
doi: 10.1016/j.physbeh.2018.12.019 URL |
| [44] |
Haroon K, Sadia P, Antoni S, et al. Current standing of plant derived flavonoids as an antidepressant[J]. Food and Chemical Toxicology, 2018,119:176-188.
doi: 10.1016/j.fct.2018.04.052 URL |
| [45] | Olsen H T, Stafford G I, Staden J, et al. Isolation of the MAO-inhibitor naringenin from Mentha aquatica L[J]. J.Ethnopharmacol, 2008,117:500-502. |
| [46] |
Guan L P, Liu B Y. Antidepressant-like effects and mechanisms of flavonoids and related analogue[J]. European Journal of Medicinal Chemistry, 2016,121:47-57.
doi: 10.1016/j.ejmech.2016.05.026 URL |
| [47] | Carly E, Masaya F, Ryuji K, et al. Novel cannabis flavonoid, cannflavin A displays both a hormetic and neuroprotective profile against amyloid β-mediated neurotoxicity in PC12 cells: Comparison with geranylated flavonoids, mimulone and diplacone[J]. Biochemical Pharmacology, 2019,169:e113609. |
| [48] |
Citti C, Braghiroli D, Vandelli M A, et al. Pharmaceutical and biomedical analysis of cannabinoids: A critical review[J]. Journal of pharmaceutical and biomedical analysis, 2018,147:565-579.
doi: 10.1016/j.jpba.2017.06.003 URL |
| [49] |
Mesquita E, Monteiro M. Simultaneous HPLC determination of flavonoids and phenolic acids profile in Pêra-Rio orange juice[J]. Food Research International, 2018,106:54-63.
doi: 10.1016/j.foodres.2017.12.025 URL |
| [50] |
Wieland P, Matteo P. 1H NMR and HPLC/DAD for Cannabis sativa L. chemotype distinction, extract profiling and specification[J]. Talanta, 2015,140:150-165.
doi: S0039-9140(15)00122-8 pmid: 26048837 |
| [51] | Baskaran X, Geo A, Parimelazhagan T, et al. Antioxidant, anti-inflammatory activities and HPLC quantification of flavonoids in Pteris tripartita Sw. a critically endangered medicinal fern from India[J]. Biocatalysis and Agricultural Biotechnology, 2019,21:e101304. |
| [52] |
Vanhoenacker G, Rompaey P, Keukeleire D, et al. Chemotaxonomic features associated with flavonoids of cannabinoid-free cannabis (Cannabis sativa subsp. sativa L.) in relation to hops (Humulus lupulus L.)[J]. Natural Product Letters, 2002,16(1):57-63.
pmid: 11942684 |
| [53] | Li M, Hou X F, Zhang J, et al. Applications of HPLC/MS in the analysis of traditional Chinese medicines[J]. Journal of Pharmaceutical Analysis, 2011,2(1):81-91. |
| [54] |
André V, Pieter V, Harald P. Recent advances and trends in the liquid-chromatography-mass spectrometry analysis of flavonoids[J]. Journal of Chromatography A, 2016,1430:16-78.
doi: 10.1016/j.chroma.2015.11.077 URL |
| [55] | Ramesh K. Application of HPLC and ESI-MS techniques in the analysis of phenolic acids and flavonoids from green leafy vegetables (GLVs)[J]. Journal of Pharmaceutical Analysis, 2017,6(7):349-364. |
| [56] | March R, Brodbelt J. Analysis of flavonoids: tadem mass spectrometry, computational methods, and NMR[J]. J.Mass Spectrom, 2008,43:1581-1617. |
| [57] |
Mohamed M R, Mahmoud A E, Desmond S, et al. Non-cannabinoid constituents from a high potency Cannabis sativa variety[J]. Phytochemistry, 2008,69:2627-2633.
doi: 10.1016/j.phytochem.2008.07.010 pmid: 18774146 |
| [58] | Veena S, Pracheta J. Extraction, isolation and identification of flavonoid from Euphorbia neriifolia leaves[J]. Arabian Journal of Chemistry, 2017,4(10):509-514. |
| [59] | Ushasree M V, Eun Y L. Flavonoids, terpenoids, and polyketide antibiotics: Role of glycosylation and biocatalytic tactics in engineering glycosylation[J]. Biotechnology Advances, 2020,41:e107550. |
| [1] | 梁俊芬, 张磊, 张辉玲, 周灿芳, 万忠. 改革开放以来广东农民收入变化特征及未来选择[J]. 中国农学通报, 2022, 38(6): 149-157. |
| [2] | 张勇, 徐智, 高丽芳, 邓亚琴, 王瑞雪, 王宇蕴. 有机类肥料部分替代化肥影响新垦红壤生菜地产量因素的研究[J]. 中国农学通报, 2022, 38(5): 79-85. |
| [3] | 李锐, 尚霄, 尚春树, 常利芳, 闫蕾, 白建荣. SSR荧光检测解析224份山西玉米自交系的遗传结构与遗传关系[J]. 中国农学通报, 2022, 38(5): 9-16. |
| [4] | 沙月霞, 黄泽阳, 魏照清. 生物菌剂撒施对宁夏石嘴山盐碱地微生物群落结构的影响[J]. 中国农学通报, 2022, 38(34): 82-90. |
| [5] | 宋应芳, 洪黄熙, 李卓丽, 程志勇, 张李香. 茶皂素控害及其增效作用研究进展[J]. 中国农学通报, 2022, 38(31): 119-124. |
| [6] | 马艳芝, 齐珂佳, 王向东. 干燥方法对‘墨红’玫瑰品质的影响[J]. 中国农学通报, 2022, 38(31): 142-146. |
| [7] | 李政璞, 佟静, 王素娜, 李炎艳, 王丽萍, 梁浩, 武占会. 光周期对植物工厂水芹产量和品质的影响[J]. 中国农学通报, 2022, 38(31): 38-42. |
| [8] | 刘琪, 高志强, 杨珍平, 乔月静. 合理氮肥用量改善冬小麦土壤耕层细菌群落结构及理化性质研究[J]. 中国农学通报, 2022, 38(30): 77-84. |
| [9] | 姜玉琴, 谢先进, 黄达. 耕地质量对耕地生产力的影响[J]. 中国农学通报, 2022, 38(3): 75-80. |
| [10] | 晁赢, 付钢锋, 阎祥慧, 杭中桥, 杨全刚, 王会, 潘红, 娄燕宏, 诸玉平. 有机肥对作物品质、土壤肥力及环境影响的研究进展[J]. 中国农学通报, 2022, 38(29): 103-107. |
| [11] | 韩文浩, 颜振敏, 吴艳兵, 蔡光辉, 赵莉蔺, 李薇, 陈增龙. 新型吡唑酰胺类农药吡噻菌胺的研究进展[J]. 中国农学通报, 2022, 38(24): 124-130. |
| [12] | 郭文, 代希茜, 莫楠, 张应青, 余晨, 田江, 耿智德, 李露. 东盟国家大豆种植及其大豆产品进出口结构分析[J]. 中国农学通报, 2022, 38(23): 156-164. |
| [13] | 崔雪娇, 佟潇禹, 张彦龙, 曾伟民. 刺五加果多糖ASPF的结构表征及其体外抗肺癌活性研究[J]. 中国农学通报, 2022, 38(22): 157-164. |
| [14] | 乔绪强, 郭婷婷, 杨炳松, 李建召, 梁美霞. 苹果组培苗移栽过程中根茎叶解剖结构变化[J]. 中国农学通报, 2022, 38(22): 49-54. |
| [15] | 王丽学, 韩静, 陈龙宾, 余新越, 刘景喜, 马毅, 霍文娟. 甲酸和木醋液对苜蓿青贮细菌群落结构的影响[J]. 中国农学通报, 2022, 38(2): 92-101. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||